How cells get rid of excess gene products
Cellular disposal at the nuclear membrane: An international team led by Sigurd Braun (until recently group leader at the BMC, Physiological Chemistry) has investigated the degradation routes of ribonucleic acid (RNA) in the cell nucleus. This study was part of the Collaborative Research Center 1064 "Chromatin Dynamics" and was published in the journal Nature Structural & Molecular Biology last September. Sigurd Braun recently moved to the Justus Liebig University of Giessen as part of a Heisenberg professorship.
Gene expression controls the global synthesis of proteins that perform distinct tasks in the cell. This happens by transcribing genes and making copies in the form of so-called messenger RNAs (mRNA). Subsequently, mRNA molecules are exported from the nucleus and translated into proteins. Multicellular organisms, such as humans, have a large number of different cells, each with a different set of proteins. Therefore, the supply of mRNAs must be tightly controlled.
One way to regulate the amount of mRNAs is through their targeted degradation. Inside the nucleus, this happens via a degradation machinery called nuclear exosome, which also degrades other, so-called non-coding RNAs (ncRNA). To manage the turnover of this broad spectrum of RNAs, the exosome works together with adapter factors that mediate the recognition and transfer of the various RNA molecules to the core degradation machinery. However, where exactly recognition and degradation takes place in the nucleus and how this is coordinated is poorly understood.
Together with their international partners, Sigurd Braun's team demonstrated that different routes exist for RNA degradation inside the nucleus. One of these degradation pathways involves the protein Lem2, which is part of the nuclear envelope and found in all eukaryotes from unicellular yeast to humans.
Cells lacking Lem2 accumulate ncRNA and mRNA, which are normally degraded by the exosome. "This was the first hint for us that the RNA degradation pathway is defective," explains Sigurd Braun. By dissecting the molecular mechanism, the researchers discovered that Lem2 is indeed part of the exosome degradation pathway and directly binds to one of its adapter factors, mediating its recruitment to the nuclear envelope. They also found that RNA molecules degraded by the nuclear exosome are often located at the nuclear membrane. Conversely, when Lem2 is missing, these RNAs are not properly recognized by the adapter factors, resulting in their accumulation. All these findings indicate that Lem2 plays a central role in the degradation pathway, coordinating the interaction between RNA recognition and transfer to the exosome at the nuclear membrane.
But why is it important to anchor the degradation machinery at the nuclear membrane? "Recruitment to the nuclear membrane leads to a concentration of degradation factors, which would otherwise be distributed in the nucleus and less likely to find each other," says Sigurd Braun. "This probably also helps to better coordinate the individual steps from the recognition of RNAs to their degradation." Interestingly, Lem2 is also involved in other tasks within the nucleus, such as keeping retrotansposons in check, also known as "jumping genes", which pose a threat to the integrity of the genome. How Lem2 performs these different tasks at the nuclear membrane remains an exciting question that Sigurd Braun's team seeks to address in future studies.
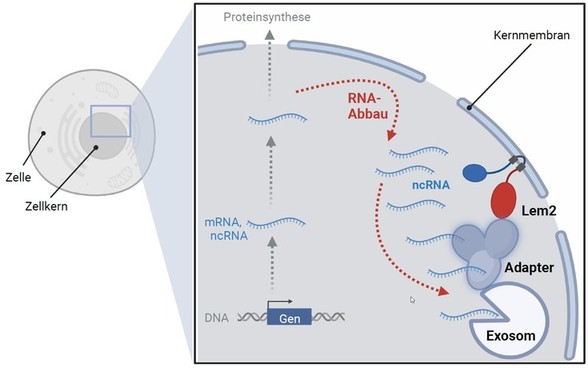
Model of the function of Lem2 in RNA degradation
Publication: The inner nuclear membrane protein Lem2 coordinates RNA degradation at the nuclear periphery. L. Martín Caballero, M. Capella, R.R. Barrales et al. Nat Struct Mol Biol 29, 910–921 (2022).

